Water

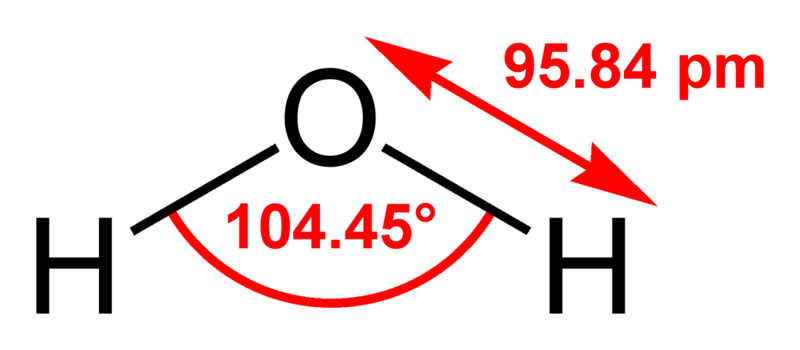
Water (H2O, HOH) is the most abundant molecule on Earth, composing 70-75% of the Earth's surface as liquid and solid state in addition to being found in the atmosphere as a vapor. It is in dynamic equilibrium between the liquid and vapor states at standard temperature and pressure. At room temperature, it is a nearly colorless, tasteless, and odorless liquid. Many substances dissolve in water and it is commonly referred to as the universal solvent; because of this, water in nature and in use is rarely clean, and may have some properties different than those in the laboratory. However, there are many compounds that are essentially, if not completely, insoluble in water. Water is the only common, pure substance found naturally in all three states of matter.
The water cycle (known scientifically as the hydrologic cycle) refers to the continuous exchange of water within the hydrosphere, between the atmosphere, soil water, surface water, groundwater, and plants.
Earth's approximate water volume (the total water supply of the world) is 1,360,000,000 km3 (326,000,000 mi3). Of this volume:
* 1,320,000,000 km3 (316,900,000 mi3 or 97.2%) is in the oceans
* 25,000,000 km3 (6,000,000 mi3 or 1.8%) is in glaciers and icecaps
* 13,000,000 km3 (3,000,000 mi3 or 0.9%) is groundwater.
* 250,000 km3 (60,000 mi3 or 0.02%) is fresh water in lakes, inland seas, and rivers.
* 13,000 km3 (3,100 mi3 or 0.001%) is atmospheric water vapor at any given time.
Mpemba effect
The Mpemba effect is the surprising phenomenon whereby hot water can, under certain conditions, freeze sooner than cold water, even though it must pass the lower temperature on the way to freezing. However, this can be explained with evaporation, convection, supercooling, and the insulating effect of frost.
History
In 1742, Anders Celsius defined the Celsius temperature scale with the freezing point of water at 100 degrees and the boiling point at standard atmospheric pressure at 0 degrees. The scale was reversed in 1744.
In 1724, Gabriel Fahrenheit defined a temperature scale in which 100 degrees was set at body temperature (now accepted as 98.6 degrees) and 0 degrees at the temperature at which equal parts of salt and water melt.
The first scientific decomposition of water into hydrogen and oxygen, by electrolysis, was done in 1800 by William Nicholson, an English chemist.
Gilbert Newton Lewis isolated the first sample of pure heavy water in 1933.
Quoted from Wikipedia
Next

